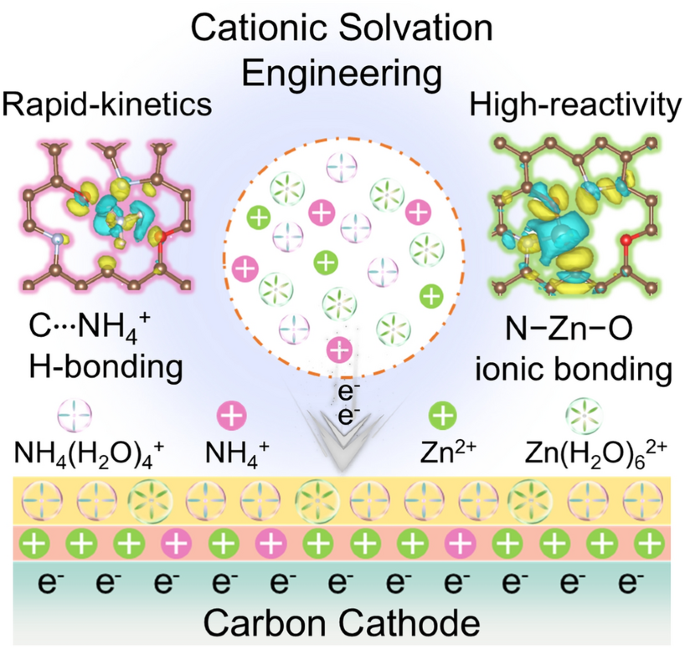
Compared with Zn2+, the current mainly reported charge carrier for zinc hybrid capacitors, small-hydrated-sized and light-weight NH4+ is expected as a better one to mediate cathodic interfacial electrochemical behaviors, yet has not been unraveled. Here we propose an NH4+-modulated cationic solvation strategy to optimize cathodic spatial charge distribution and achieve dynamic Zn2+/NH4+ co-storage for boosting Zinc hybrid capacitors. Owing to the hierarchical cationic solvated structure in hybrid Zn(CF3SO3)2–NH4CF3SO3 electrolyte, high-reactive Zn2+ and small-hydrate-sized NH4(H2O)4+ induce cathodic interfacial Helmholtz plane reconfiguration, thus effectively enhancing the spatial charge density to activate 20% capacity enhancement. Furthermore, cathodic interfacial adsorbed hydrated NH4+ ions afford high-kinetics and ultrastable C‧‧‧H (NH4+) charge storage process due to a much lower desolvation energy barrier compared with heavy and rigid Zn(H2O)62+ (5.81 vs. 14.90 eV). Consequently, physical uptake and multielectron redox of Zn2+/NH4+ in carbon cathode enable the zinc capacitor to deliver high capacity (240 mAh g−1 at 0.5 A g−1), large-current tolerance (130 mAh g−1 at 50 A g−1) and ultralong lifespan (400,000 cycles). This study gives new insights into the design of cathode–electrolyte interfaces toward advanced zinc-based energy storage.