Chiral phosphine-containing compounds are widely found in natural products and pharmaceutically active molecules, and are also important chiral catalysts and ligands, greatly promoting the development of organic chemistry and playing an important role in asymmetric catalysis. With the continuous development and intensive research of metal catalysis in recent years, the important role of chiral ligands in chemical synthesis, medicine, materials, fine chemicals and other fields has been highlighted. However, chiral phosphine-containing compounds are synthesized by very limited means and mostly rely on chiral resolution. However, the asymmetric carbon-carbon bonding reaction of phosphine-containing compounds provides a very facile approach.
Professor Xu Tao's research team has been dedicated to the asymmetric synthesis of phosphine compounds. Recently, the research team has made important progress in the asymmetric synthesis of α-aryl phosphates via nickel and photoredox catalysis, and released their relevant research outcomes by publishing a paper titled Modular and Facile Access to Chiral α-Aryl Phosphates via Dual Nickel- and Photoredox-Catalyzed Reductive Cross-Coupling inJournal of the American Chemical Society, the most important academic journal in the international chemistry community.
Asymmetric carbon-carbon bonding has always been an important subject in organic chemistry research, and coupling reaction has become one of the important ways thanks to its precise bonding. Meanwhile, nickel catalysts are widely used in asymmetric carbon-carbon coupling reactions, leveraging their great advantages in sp3carbon bonding. Several common research strategies have been developed. The first is the traditional asymmetric coupling, a kind of bonding reaction based on electrophilic reagents and nucleophilic reagents. The second is the asymmetric reduction coupling strategy, which is to realize the facile cross-coupling reaction of two electrophilic reagents with the help of metal reducing agents such as zinc and manganese, without preparing organometallic reagents in advance. Some direct asymmetric coupling reactions can also be realized by virtue of thephotoredox and nickelsystem.
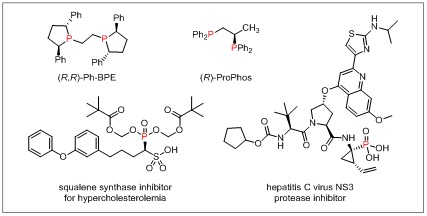
In order to further improve asymmetric coupling reactions, Professor Xu Tao's research team has applied the nickel and photoredox catalytic system to asymmetric reduction coupling reactions, resulting in a series of bonding reactions, and particularly focused on the conversions that cannot be realized in the traditional strategies, and developed several new types of asymmetric reduction reactions via dual nickel and photoredox catalysis. In addition to the α-arylation reactions realized with borate, trifluoromethyl and other structures in the previous research, the research team released a new method for the preparation of chiral α-aryl phosphates, to further expand the research system. The chiral α-aryl phosphates are synthesized by asymmetric reduction cross-coupling of aryl iodides and α-bromophosphate under the dual nickel and photoredox catalytic conditions, with good functional group compatibility and excellent enantioselectivities, and are applied to the development of a new chiral phosphine ligand and the synthesis of enzyme inhibitor derivatives.

It is worth mentioning that conversion cannot be realized in the reaction, with the traditional metal reducing agent and the organic reducing agent, which demonstrates the unique advantage of the asymmetric reduction coupling reaction system via nickel and photoredox catalysis, in comparison with the traditional strategy. Revealing the reaction mechanism through in-depth study, the research team further proved the advantages of the reaction system and explained the possible reasons for the failure of the traditional reduction coupling conditions. Li Yuqiang from Central South University carried out DFT calculations for the reaction, and theoretically demonstrated the reaction process and determining steps of product configuration.
Wang Hepan, a doctoral candidate of our school, contributed as the first author, and Professor Xu Tao as the corresponding author. The research work was funded by the National Natural Science Foundation of China and the National Youth Talent Program.