In nature, a series of products from small molecules to biomolecules can be obtained through biological carbon sequestration and carbon dioxide reduction reactions. Although multi-carbon products can be obtained by artificial electrocatalytic CO2reduction reaction at present, C3+ products with multiple functional groups (such as C-N bond and C=O bond), especially biological molecules, still cannot be obtained.In response to this problem, the research team of Professor Han Lu of our school, Professor Che Shunai and Professor Zhou Zhongyue of Shanghai Jiao Tong University and Professor Liu Zhipan of Fudan University worked together to tackle the critical point.Recently, they artificially synthesized amino acids by applying chiral inorganic copper nanomaterials to the electrocatalytic reduction of carbon dioxide for the first time, and the research outcome "Synthesis of Amino Acids by Electrocatalytic Reduction of CO2on Chiral Cu Surfaces" was published in Chem, a well-known journal of Cell Press.
Through the research, C3+enantiomeric amino acids containing C-N bonds were obtained by electrocatalytic reduction of carbon dioxide for the first time. It is found that the chiral crystal plane limits the configuration change of C3+intermediates on the catalyst surface, thereby reducing the reaction energy barrier of carbon dioxide reduction synthesis of C3+products. Although the main amino acid product is serine, the formation of a variety of amino acids and urea is also observed in the reaction, which indicates that chiral crystal plane electrocatalysis has broad application prospects for the synthesis of biomolecules. In addition, the introduction of chiral crystal planes into catalytic systems found in the research is also a powerful way to improve the intrinsic activity of catalytic materials for synthesizing C3+products.
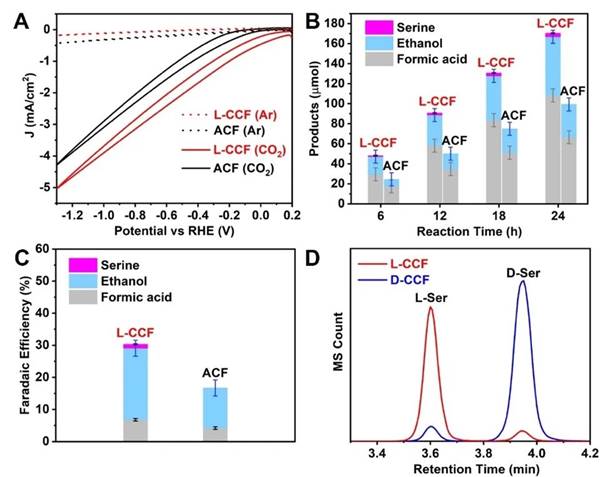
In the research, the main amino acid product synthesized by artificial electrocatalysis is serine, and its enantiomer excess value (ee%) can exceed 90%. Moreover, it was further found that the mechanism of amino acid formation was attributed to the change of the chiral crystal plane on the surface of chiral inorganic copper nanofilm from thermodynamic and kinetic to the electrocatalytic reduction path of carbon dioxide. The research provides an important reference for the electrocatalytic reduction of carbon dioxide to synthesize multi-carbon products with high added value and chirality.
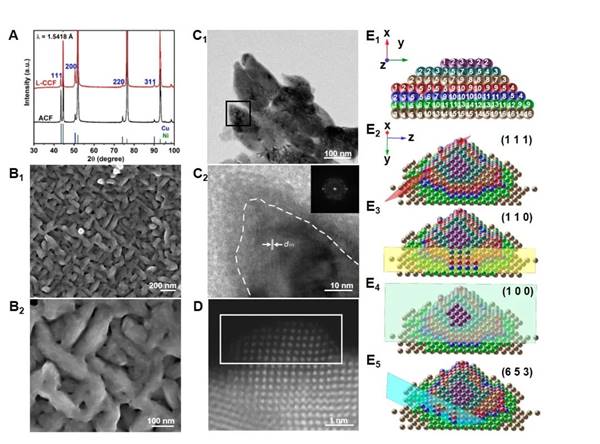
Copper and copper alloys are the main catalysts for the electrocatalytic reduction of carbon dioxide to synthesize multicarbon products, C1-C2molecules and C3-4fatty alcohols. However, CO2 reduction products containing C-N bonds are limited to C1-C2products (urea, methylamine, and ethylamine). According to experimental and theoretical reports, the atomic configuration of the catalyst surface can improve the intrinsic catalytic activity of Cu-based catalysts for electrocatalytic carbon dioxide reduction reactions by reducing the energy barrier of the reaction pathway. However, the currently reported enhancement of catalytic activity by copper surfactant sites is only observed in C1-2molecules and n-propanol.
Based on this, the paper applies chiral inorganic copper nanomaterials as electrocatalytic carbon dioxide reduction catalysts for the first time and reveals the existence of chiral nanostructures and chiral surfaces (Cu(653)S) through scanning electron microscopy, transmission electron microscopy and theoretical calculations.
The catalytic properties of the synthesized chiral inorganic copper nanofilms were further studied, and the faraday efficiency of ~3.8 (±0.6), ~58.6 (±6.5) and ~108.1 (±6.5) μmol of serine, ethanol and formic acid products can be obtained on the CV graph after 24 hours of -0.6~-1.3V vs RHE: ~1.2 (±0.2), ~22.3 (±2.5) and ~6.8 (±0.4) %. Among them, the ee% of serine can reach 94%.
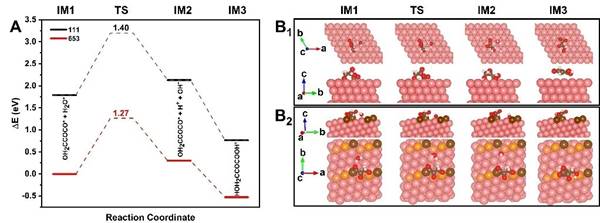
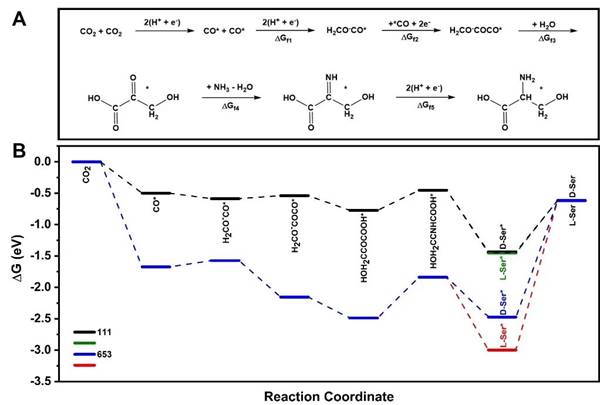
Through density functional theory (DFT) calculations, the researchers studied the pathway and mechanism of electrocatalytic reduction of carbon dioxide to serine. It is calculated that the chiral crystal plane Cu(653)Sis more thermodynamically tended to form 3-hydroxypyruvate intermediate and serine, and more inclined to form L-serine, which confirms that the chiral crystal plane structure on the surface of chiral inorganic copper nanofilm plays an important role in the formation of serine and enantiomeric selectivity.
Further DFT calculations showed that chiral crystal plane Cu(653)Scan also promote the formation of serine dynamically, and stabilize the configuration of important intermediates H2COCOCO* and 3-hydroxypyruvic acid*, thereby reducing the activation energy and reaction barrier of H2COCOCO* hydrolysis to form 3-hydroxypyruvic acid*.
The doctoral student Fang Yuxi of our school is the first author of the paper; Professor Han Lu of our school, Professor Che Shunai and Professor Zhou Zhongyue of Shanghai Jiao Tong University, and Professor Liu Zhipan of Fudan University are the corresponding authors.