One-step-excitation photocatalytic water splitting is one of the simplest and most cost-effective approach to produce hydrogen. However, the process is both thermodynamically and kinetically unfavorable. Very few semiconductive photocatalyst has achieved this catalysis based on visible light excitation. Metal halide materials has many intriguing properties for photocatalysis, such as tunable bandgaps and excellent photocarrier properties.
Honghan Fei research group has focused on synthesis of cationic metal halide building blocks for photoactive properties. They have employed anionic organic ligands to template cationic 1-D chains (Pb2X3+, Angew. Chem. Int. Ed. 2019, 7818), cationic 2-D layers (Pb2X22+, Angew. Chem. Int. Ed. 2017, 14411) and cationic 3-D frameworks (Pb4Br62+, Chem. Sci. 2018, 627). These cationic building units have high Pb:X ratio to afford high-coordinate bridging halide, thus exhibiting high moisture stability and photostability. Unfortunately, the attempts to narrow their bandgap and extend the absorption edge to visible region were not successful.
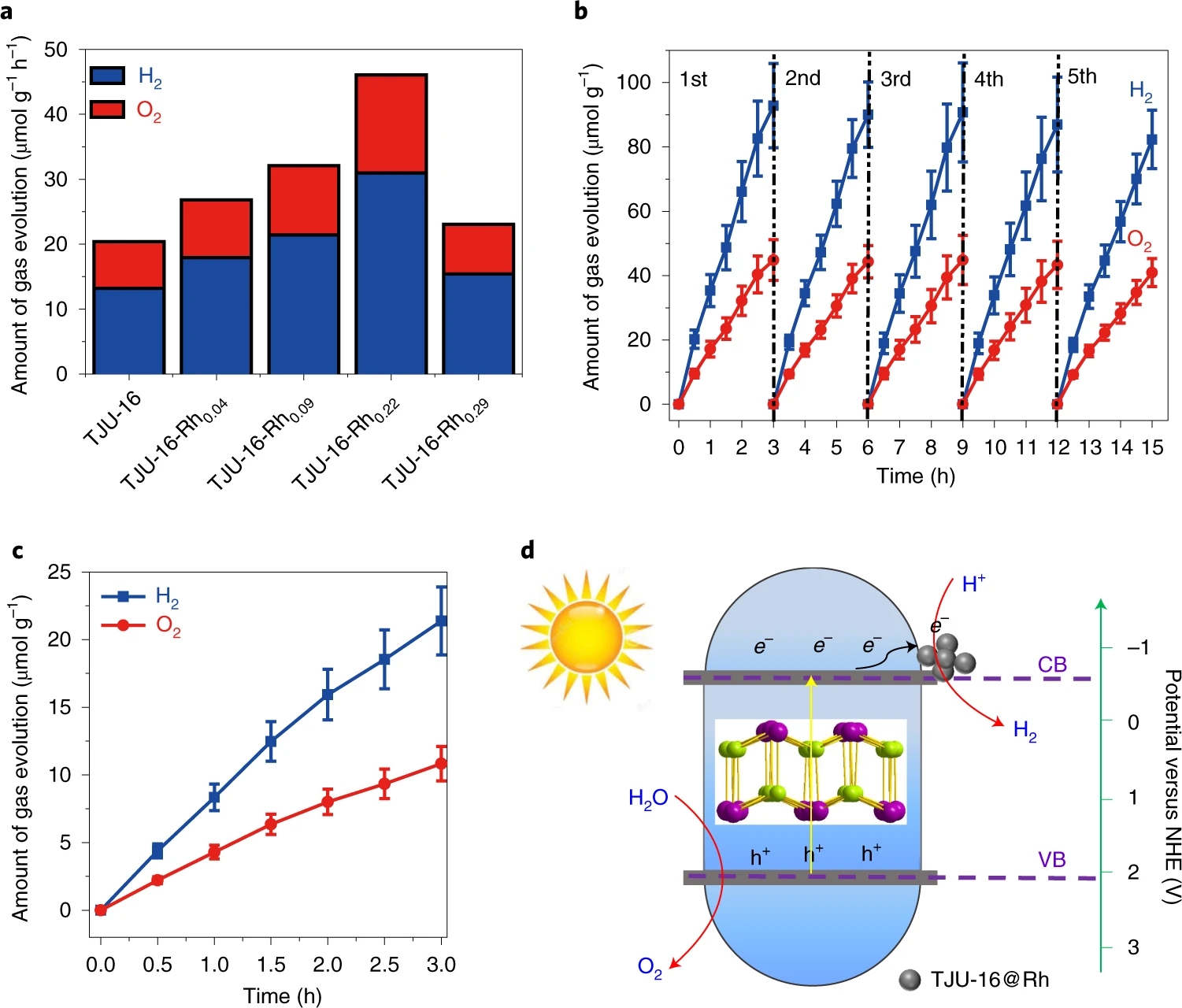
Recently, Fei group have reported a visible-light-responsive organolead iodide crystalline material [Pb8I8(H2O)3]8+ (TJU-16). Both experimental and theoretical studies indicate the high chemical stability of TJU-16 arise from the high-coordinate iodide anions located in the inner spaces of each cationic layer. The material not only has a conduction band and valence band straddling the water-splitting redox potential, but also has a narrow bandgap of 2.74 eV. Hall effect measurement and transient-state photoluminescence study indicate long photocarrier diffusion distance of 0.4~1.4 μm, superior to many metal oxide semiconductors. Under simulated solar illumination, the material steadily performs photocatalytic overall water splitting without use of any sacrificial agent or co-catalyst. The sustained photocatalytic activity is 2.4-fold enhanced when combined with 0.22 wt.‰ Rh co-catalyst, up to 5 cycles and 57 h continuous irradiation. Even decreasing the illumination intensity to 20 mW cm-2, TJU-16 still catalyzes the water splitting with a calculated overall solar-to-hydrogen conversion efficiency of 0.014%.
Paper: https://www.nature.com/articles/s41929-020-00543-4

 Home
>
Content
Home
>
Content