A facile and efficient synthetic reaction with high atom economy developed by Prof. Yanghui Zhang’s group Atom economy (atom efficiency) is the conversion efficiency of a chemical process in terms of all atoms involved and the desired products produced. In chemical reactions, the key to reduce the formation of chemical wastes is to improve atom economy. Atom-economical reactions can maximize the utilization of resource and reduce environmental pollution caused by chemial reaction. In an ideal atom-economical reaction, all the atoms of starting materials are transformed into products, and no byproducts or chemial wastes are generated and produce zero pollution. The “atom-economy” in chemical reactions is one of the core focuses of green chemistry and has been the hotspot in modern chemical synthesis. Prof. Yanghui Zhang’s group in School of Chemical Science and Engineering has been committed to developing simple and efficient atom-economical synthetic methods, particularly innovative organic reactions based on palladacycle complexes. In the past few years, the Zhang group has made great achievements in palladacycle chemistry, and developed a series of novel organic reactions. After the recent discovery on alkylation of palladacycle intermediate (Angew. Chem. Int. Ed. 2017, 58, 12288. DOI: 10.1002/anie.201800330), a significant progress in this field has been made again.
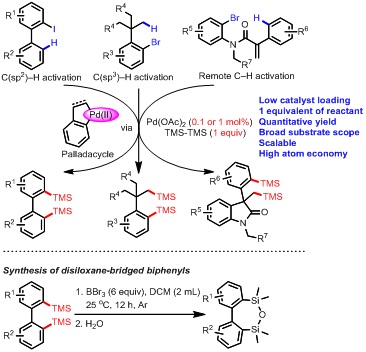
In this significant discovery, they found that palladacycles, produced via Pd-catalyzed C(sp2)–H or C(sp3)–H bond activation of arylhalides, could react with hexamethyldisilane and form disilylated arenes as the final products. Notably, almost 100% yield was achieved with only 0.1 mol % Pd-catalyst and one equivalent of hexamethylsilane. The two trimethylsilyl groups were completely transformed into the products and no byproducts were formed, so this transformation is a typical atom-economical reaction. Moreover, the reaction possesses broad substrate scope and great compatibility of various functional groups, and a range of palladacycle intermediates derived from C(sp2)–H, C(sp3)–H or remote C–H bond activation are effective. The ingenious reaction is highly facile and efficient, and represents a new type of organic reactions. The products have important applications in materials science and medicinal chemistry. Furthermore, the products could be transformed into disiloxane-bridged biphenyls, which have great application potentials in OLED materials. The research has been published as “Palladium-Catalyzed C–H Silylation through Palladacycles Generated from Aryl Halides”in Angewandte Chemie, International Edition, (Angew. Chem. Int. Ed. 2018, DOI: 10.1002/anie.201800330). Prof. Yanghui Zhang is the sole corresponding author, and MSc student Ailan Lu and PhD student Xiaoming Ji are listed as the first authors. The research was supported by NSFC (21372176、21672162). |
