The synthesis of secondary α,α-dialkyl boronates has always drawn great interest from scientists since they are widely used due to their great versatility in conversion. Primary α,α-dialkyl boronates can be prepared facilely by the hydroboration method of terminal olefins, but there are obvious regioselectivity and other adverse trends when this method is applied to the synthesis of secondary α,α-dialkyl boronates. Such compounds are generally synthesized by borylation reaction of alkylates or alkylation reaction of boron-containing compounds, both of which require the introduction of alkyl and boron functional groups in steps, resulting in cumbersome processes in multiple steps for preparation and purification. Comparatively speaking, the simultaneous introduction of alkyl and boron functional groups is more advantageous in synthesis. Although some methods of 1,2-alkylboration or 1,1-alkylboration of olefins have been reported recently, it is still necessary to develop an approach for efficient synthesis of secondary α,α-dialkyl boronates.
Professor Xu Tao's research team in our school has realized the efficient synthesis of secondary α,α-dialkyl boronates with the widely existing or readily available aldehydes as reaction precursors and via the deoxygenative alkylboration strategy, creating a new idea for synthetic technology. They published a paper titled Modular and Fast Synthesis of Versatile Secondary α,α-Dialkyl Boronates via Deoxygenative Alkylboration of Aldehydes inAngewandte Chemie International Edition(2022, e202214213) online, a famous international academic journal in the chemistry community to release their outcomes.

The researchers applied the photoredox and nickel dual catalysis strategy to sp3-sp3deoxygenative reduction coupling of intermediate C-O bond, realizing deoxygenative alkylboration of aldehydes in a one-pot synthesis process. Under extremely mild reaction conditions and in good functional group compatibility, the reaction via deoxygenative difunctionalization ofaldehydescan be realized by one-step product conversion.
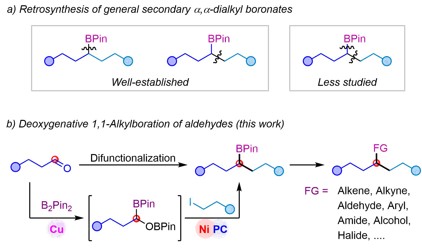
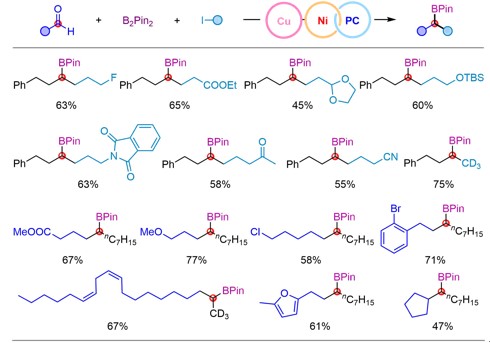
The researchers explored the scope of application of such a kind of reaction under optimal conditions, and the results showed good compatibility with various functional groups such as ether, ester, ketone, amide, acetal, fluorine, chlorine, bromine and trifluoromethyl. In addition, the mild reaction conditions also make it possible to use this method for late-stage modification of complex molecules.
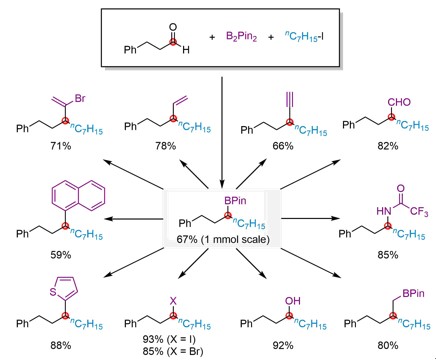
It is worth mentioning that such secondary α,α-dialkyl boronates can be used to build up a series of structures such as aldehyde, alkyl, alkenyl, alkynyl, halogen, alcohol, amine, heterocycle and aryl functional groups via synthesis of carbon-carbon bonds and carbon-heteroatom bonds in one step. Playing a synthetic transfer role, this method contributes to difunctionalization of aldehydes in only two steps, highlighting the great potential of this method in organic synthesis. Revealing the reaction mechanism through in-depth study, the research team proposed the possible reaction cycle.
A new reaction via deoxygenative alkylboration of aldehydes has been developed under this research, which provides a very simple and universal solution for the preparation of secondary α,α-dialkyl boronates. More importantly, due to the versatility in conversion of the product, a series of deoxygenative difunctionalizations of aldehydes can be realized in only a two-step reaction with this method, opening up more opportunities for fast and efficient building of complex molecules.
Xu Wenhao, a doctoral candidate of our school, contributed as the first author of the paper, and Professor Xu Tao as the corresponding author. The research work was funded by the National Natural Science Foundation of China.