Important Progress in the Treatment of Triple Negative Breast Cancers by Prof. Shi’s Interdisciplinary Collaborative Research
Breast cancer is the most common malignant tumor and has become a significant cause of cancer-related deaths in females. Triple negative breast cancer (TNBC) has a high recurrence and metastasis rate, a short survival period after recurrence and metastasis, a poor prognosis, and easy resistance to chemotherapy. Currently, TNBC is mainly treated by surgical resection, chemotherapy and radiotherapy, and no targeted therapy of estrogen-progesterone receptor antagonist and anti-her-2 is available. Therefore, how to improve the therapeutic effect of TNBC is a difficult problem in clinical breast cancer treatment. Prof. Shuo Shi cooperated with Prof. Chunyan Dong research group, and then proposed a new strategy of combining chemotherapy and immunotherapy with photothermal and photodynamic technology to treat TNBC. This work has been published in Advanced Materials recently.
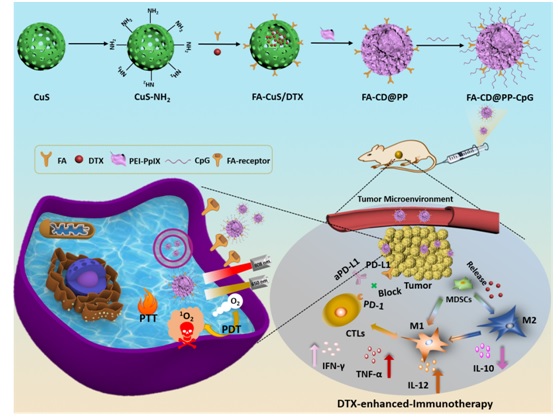
In recent years, owing to the approval of the cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) antibody ipilimumab, the programmed cell death protein 1 (PD-1) antibody nivolumnab and its ligand (PD-L1) antibody atezolizumab, cancer immunotherapy has brought a revolution in cancer therapy. Compared with the excellent effects of immunotherapy in melanoma, non-small-cell lung cancer and other malignant tumors, immunotherapy works less effectively on breast cancer, mainly because of the efficient filtration of lymphocytes and myeloid-derived suppressor cells (MDSCs). MDSCs induce T cells dysfunction and further reduce proliferation, then increase T cells apoptosis of antigen-specific CD8+ T cells, leading to immune suppression. Tumor-related MDSCs can be divided into two subsets: M1 [marker: CCR7] and M2 [marker: mannose receptor (MR) and IL-10], which also named as M2 tumor-associated macrophages (TAMs). As we know, M1-type macrophages are generally recognized as potent effector cells, which produce various pro-inflammatory cytokines (e.g., IL-12 and TNF-α) and inducible nitric oxide synthase (iNOS), then kill tumor cells. In contrast, M2-Type macrophages can secrete IL-10, IL-4, and IL-13 to promote tumor angiogenesis. In traditional view, chemotherapeutics causes a suppressive effect on immune system for patients. Recent research revealed that low dosage of traditional chemotherapeutics could induce immunogenic death of tumor cells or engage immune effector mechanisms to stimulate tumor-specific immune responses. Especially, docetaxel (DTX) can decrease MDSCs proportion and induce MDSCs polarizing toward an M1-like phenotype, not M2-like phenotype. As an immunoadjuvant, CpG can specifically bind with Toll-like receptor 9 in plasmacytoid antigen presenting cells (APCs) (including dendritic cells and macrophages), thus stimulate an immune response to produce helper T cell 1 (Th1) and a proinflammatory cytokine (such as tumor necrosis factor alpha, TNF-α; IL-12 (where IL stands for interleukin)). IL-12, as one of most effective stimulator of CTLs, could promote maturity of CTLs and infiltration in tumor sites. Simultaneously, activated CTLs could secrete interferon gamma (IFN-γ) to kill tumor cells.
In this work, mesoporous CuS nanoparticles were chosen as the drug nanocarrier and PTT agents owing to the high photothermal conversation efficiency and excellent biocompability. Then, the surface modification with tumor target ligand folic acid (FA) was favorable for improving active delivery of nanoparticles, further enhancing the transport efficiency of DTX at tumor sites through targeting effect of FA to overexpressed FA receptors on the tumor cells. To improve the water solubility of nanoparticles and further realize CpG delivery, PEI-PpIX (polyethylenimine–protoporphyrin IX) conjugates and CpG were anchored alternatively to fabricate FA-CuS/DTX@PEIPpIX-CpG (denoted as FA-CD@PP-CpG) nanocomposite. Combination of FA-CD@PP-CpG with aPD-L1 enhanced the infiltration of CTLs, which was favorable of aPD-L1 to block the PD-1/PD-L1 checkpointblockade and further prevent CTLs from dysfunction and exhaustion, thus kill cancer cells efficiently. Furthermore, DTX transported by FA-CD@PP-CpG to tumor cells could directly induce a phenotype polarization of MDSCs contributes to more generation of proinflammatory cytokines, thus resulting in enhanced immunotherapy efficacy. Taken together, this study presented a novel strategy for highly superior antitumor efficiency by combining PDT, PTT, and docetaxel-enhanced immunotherapy, which might open new avenues in breast cancer immunotherapy for clinical translation.
This work entitled “Tumor-Targeted Drug and CpG Delivery System for Phototherapy and Docetaxel-Enhanced Immunotherapy with Polarization toward M1-Type Macrophages on Triple Negative Breast Cancers” has been published in Advanced Materials whose impact factor is 25.8 on 2019. Lv Chen and Lulu Zhou are the first authors. Prof. Shuo Shi with his collaborators Prof. Chunyan Dong (Breast Cancer Center, Shanghai East Hospital, Tongji University) are the corresponding authors.
Link: https://onlinelibrary.wiley.com/doi/full/10.1002/adma.201904997

 Home
>
Content
Home
>
Content